Media Preparation and Filling Videography

Media Preparation and Filling Videography
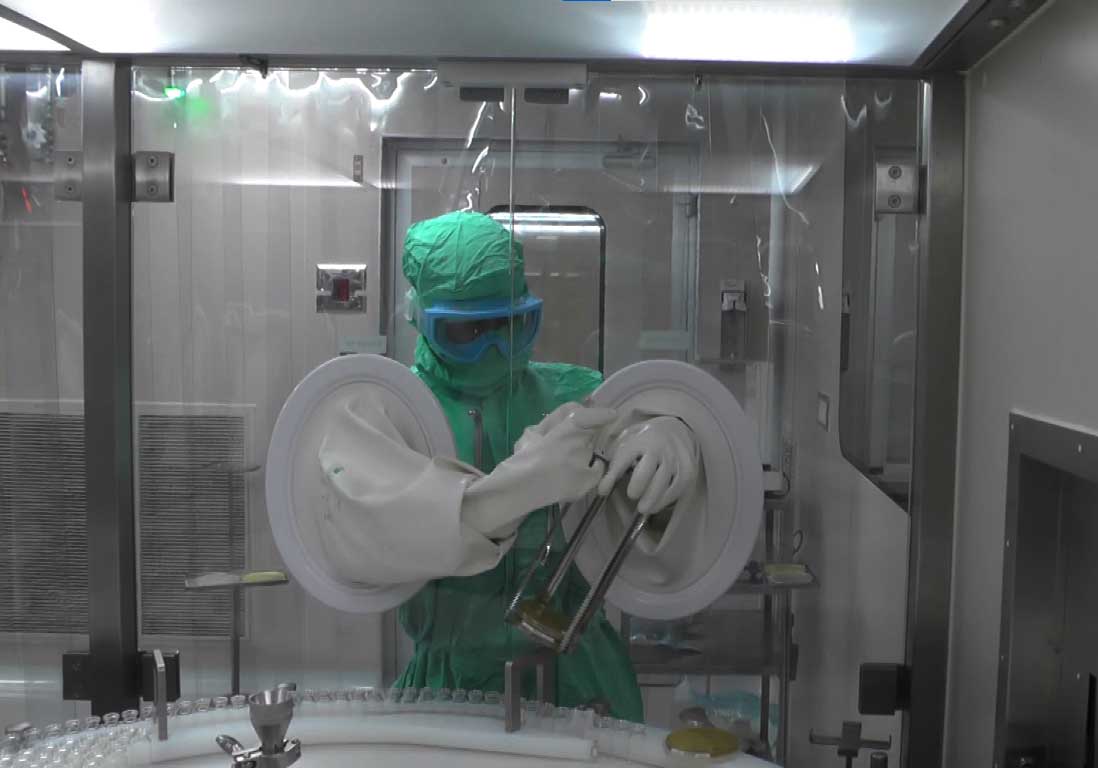
Media fill is the performance of an aseptic manufacturing procedure using a sterile microbiological growth medium in place of the drug solution.
- Factors associated with the longest permitted run on the processing line that can pose Contamination risk due to operator fatigue.
- Representative number, type, and complexity of normal interventions that occur with each run, as well as non-routine interventions and events like maintenance, stoppages, and equipment adjustments and breakdowns. Define allowable interventions
- Define grades of interventions
- Practise all interventions during media runs
- Lyophilisation
- Aseptic assembly of equipment, machines at start-up, during processing and manufacturing.
- Number of personnel and their activities
- ‘Worst case’ conditions, which typically involve the largest container with the widest mouth or small ampoules running at high speed with frequent jamming
- Timings, Days, Line speed, Size of container, number of containers Video recording of a media fill test validation maybe a helpful aide in investigating personnel behaviour that could badly affect the aseptic manufacturing process.
Video Recording during batch manufacturing & filling
Video recording of a media fill validation may be a useful aide in investigating personnel behaviour that could negatively affect the aseptic manufacturing process.
Micron uses advance multi angle camera system to perform videography without human intervention.
We provide multi angle simulation video as final proof of media fill Qualification. For generating multi angle simulation video, we use high editing software. We can visualize the videography recording remotely during media fill.
Our Services
Israel
- GMP Auditing
- GMP Training
- Pharma Microbiology
- Pharma Quality Management System
- Aseptic Processing
- Pharma Regulatory Services
- USFDA Consultant
- GMP Consultancy
- HVAC Design Consultants
- HVAC Consultant
- Cleanroom Validation Services
- Operation Theatre Validation
- Thermal Validation
- PLC Validation
- Compressed Air Validation
- Temperature Mapping
- HVAC System Validation
- HVAC System in Pharmaceutical Industry
- HVAC Validation Services
- Pharma Consultant
- Biotech Consultant
- Biopharma Consultant
- Biopharmaceutical Consultant
- Biopharma Regulatory Consultant
Saudi Arabia
- GMP Auditing
- GMP Training
- Pharma Microbiology
- Pharma Quality Management System
- Aseptic Processing
- Pharma Regulatory Services
- USFDA Consultant
- GMP Consultancy
- HVAC Design Consultants
- HVAC Consultant
- Cleanroom Validation Services
- Operation Theatre Validation
- Thermal Validation
- PLC Validation
- Compressed Air Validation
- Temperature Mapping
- HVAC System Validation
- HVAC System in Pharmaceutical Industry
- HVAC Validation Services
- Pharma Consultant
- Biotech Consultant
- Biopharma Consultant
- Biopharmaceutical Consultant
- Biopharma Regulatory Consultant
UAE
- GMP Auditing
- GMP Training
- Pharma Microbiology
- Pharma Quality Management System
- Aseptic Processing
- Pharma Regulatory Services
- USFDA Consultant
- GMP Consultancy
- HVAC Design Consultants
- HVAC Consultant
- Cleanroom Validation Services
- Operation Theatre Validation
- Thermal Validation
- PLC Validation
- Compressed Air Validation
- Temperature Mapping
- HVAC System Validation
- HVAC System in Pharmaceutical Industry
- HVAC Validation Services
- Pharma Consultant
- Biotech Consultant
- Biopharma Consultant
- Biopharmaceutical Consultant
- Biopharma Regulatory Consultant
Nigeria
- GMP Auditing
- GMP Training
- Pharma Microbiology
- Pharma Quality Management System
- Aseptic Processing
- Pharma Regulatory Services
- USFDA Consultant
- GMP Consultancy
- HVAC Design Consultants
- HVAC Consultant
- Cleanroom Validation Services
- Operation Theatre Validation
- Thermal Validation
- PLC Validation
- Compressed Air Validation
- Temperature Mapping
- HVAC System Validation
- HVAC System in Pharmaceutical Industry
- HVAC Validation Services
- Pharma Consultant
- Biotech Consultant
- Biopharma Consultant
- Biopharmaceutical Consultant
- Biopharma Regulatory Consultant
Ireland
- GMP Auditing
- GMP Training
- Pharma Microbiology
- Pharma Quality Management System
- Aseptic Processing
- Pharma Regulatory Services
- USFDA Consultant
- GMP Consultancy
- HVAC Design Consultants
- HVAC Consultant
- Cleanroom Validation Services
- Operation Theatre Validation
- Thermal Validation
- PLC Validation
- Compressed Air Validation
- Temperature Mapping
- HVAC System Validation
- HVAC System in Pharmaceutical Industry
- HVAC Validation Services
- Pharma Consultant
- Biotech Consultant
- Biopharma Consultant
- Biopharmaceutical Consultant
- Biopharma Regulatory Consultant
Bangladesh
- GMP Auditing
- GMP Training
- Pharma Microbiology
- Pharma Quality Management System
- Aseptic Processing
- Pharma Regulatory Services
- USFDA Consultant
- GMP Consultancy
- HVAC Design Consultants
- HVAC Consultant
- Cleanroom Validation Services
- Operation Theatre Validation
- Thermal Validation
- PLC Validation
- Compressed Air Validation
- Temperature Mapping
- HVAC System Validation
- HVAC System in Pharmaceutical Industry
- HVAC Validation Services
- Pharma Consultant
- Biotech Consultant
- Biopharma Consultant
- Biopharmaceutical Consultant
- Biopharma Regulatory Consultant
